Restore in hemato-oncology:
MaaT Pharma’s therapies for the treatment of acute Graft-vs-host-Disease and to improve survival in patients receiving hematopoietic stem cell transplantation. This aims to restore healthy microbiome functions to correct the impact of stressors such as antibiotics, chemotherapies.
Pioneering a full-ecosystem approach
We are developing a novel class of microbiome therapies
to address severe medical need in oncology.
MaaT Pharma’s pipeline leverages two approaches:
Restore & modulate in immuno-oncology:
MaaT Pharma’s therapies for the treatment of solid tumors aim to restore and modulate the microbiome to improve patient’s response to immune checkpoint inhibitors.
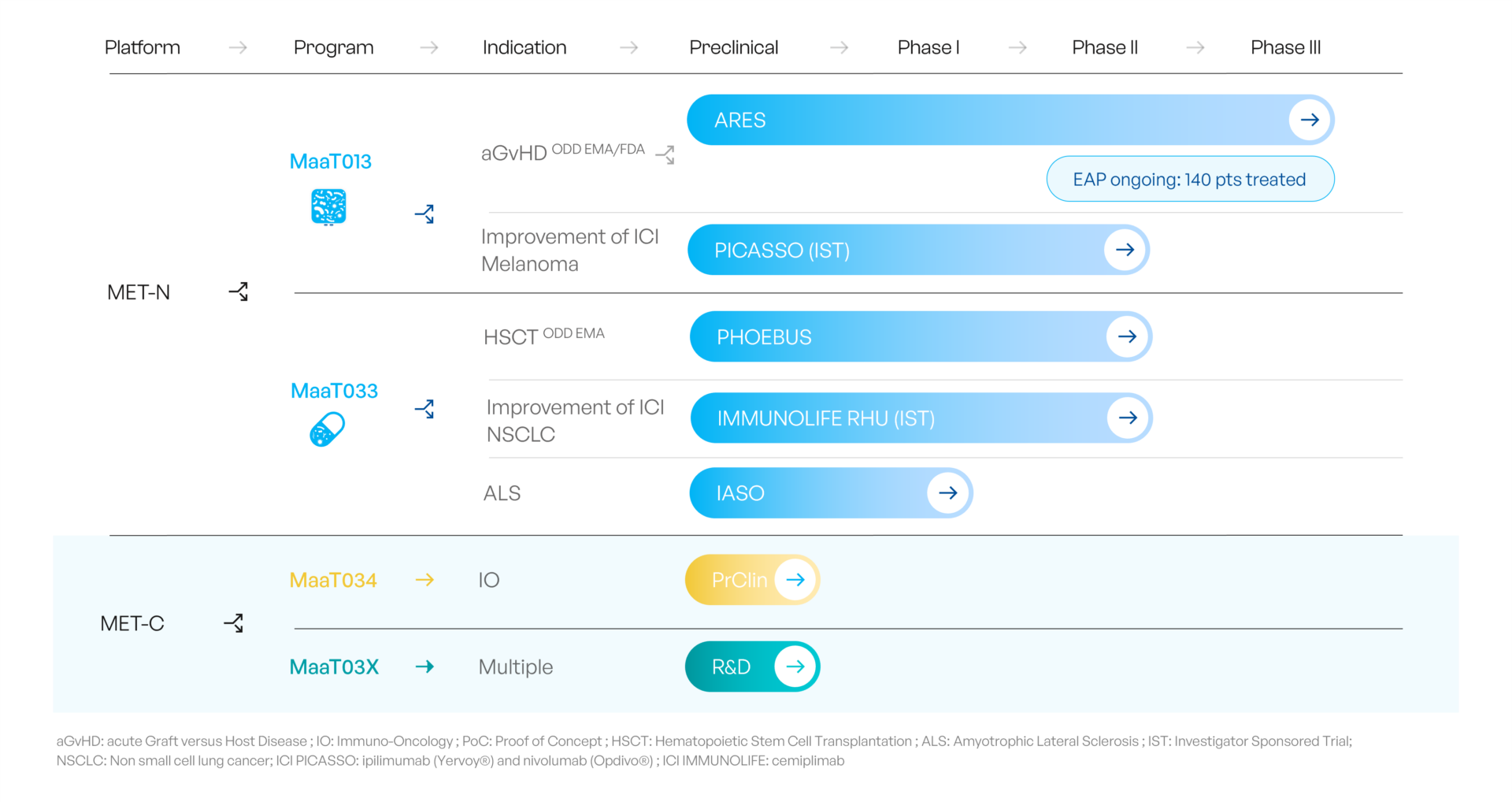
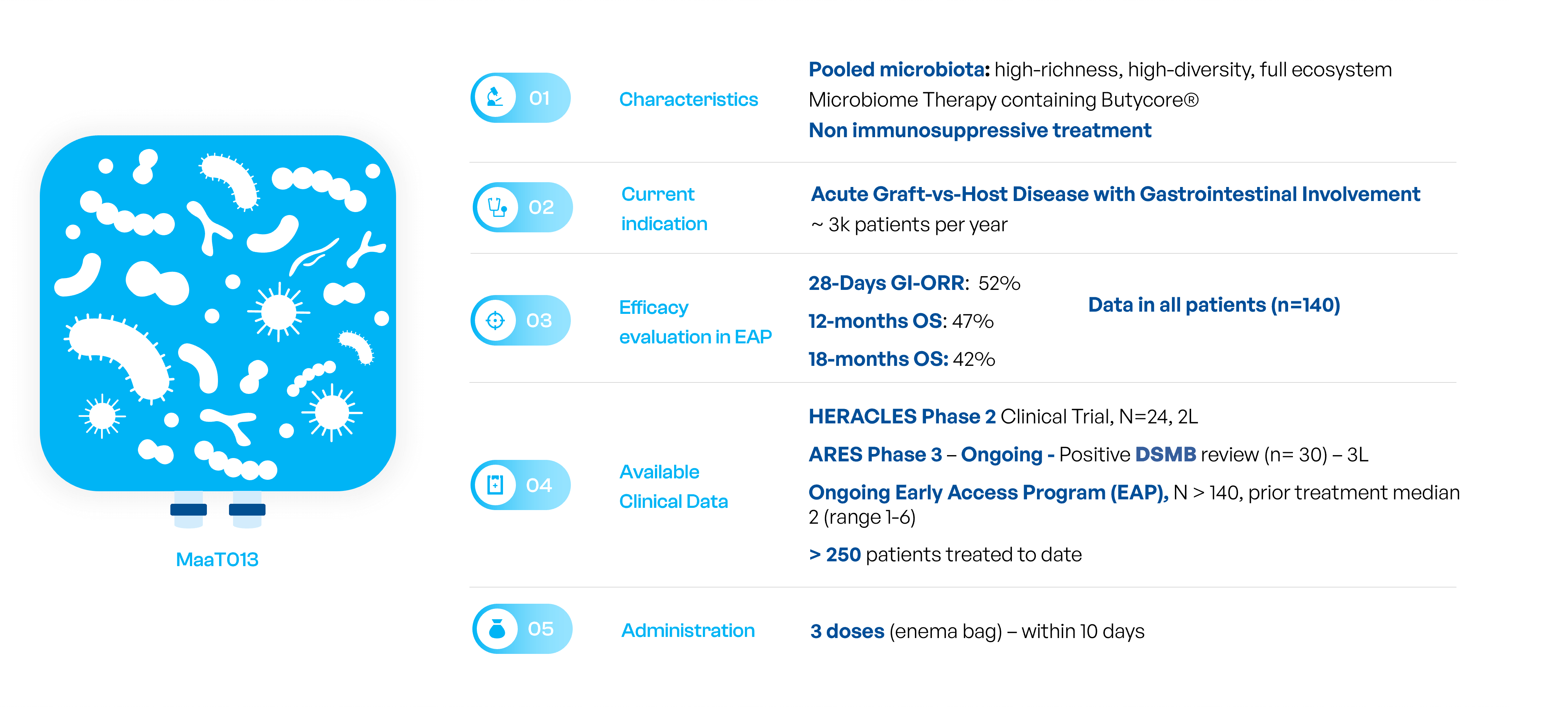
MaaT013: Treating acute Graft-vs-host-Disease
50%
70-80%
10,000
Microbiome Restoration with MaaT013: A Maximum-Density Product for Fast Engraftment in Acute Situations
MaaT013 status
MaaT013 is currently being investigated in a pivotal Phase 3 trial (ARES) in patients with acute Graft-versus-Host-Disease with gastrointestinal involvement (GI-aGvHD) who are refractory to both steroids, the standard of care first-line treatment, and to ruxolitinib used as a second-line treatment.
ARES is the first Phase 3 trial globally for a microbiome-based therapy in hemato-oncology.
Additional information:
– Clinical Trial. gov : NCT04769895
– First patient dosed in Phase 3 clinical trial ARES
– Positive Outcome Following DSMB Review for ongoing Phase 3
200
Florent Malard, M.D, Professor of Hematology Saint-Antoine Hospital – Sorbonne University, Paris, France presented the results during an oral presentation intitled “Pooled Fecal Allogenic Microbiotherapy for Refractory Gastrointestinal Acute Graft-Versus-Host Disease: Results from Early Access Program in Europe”.
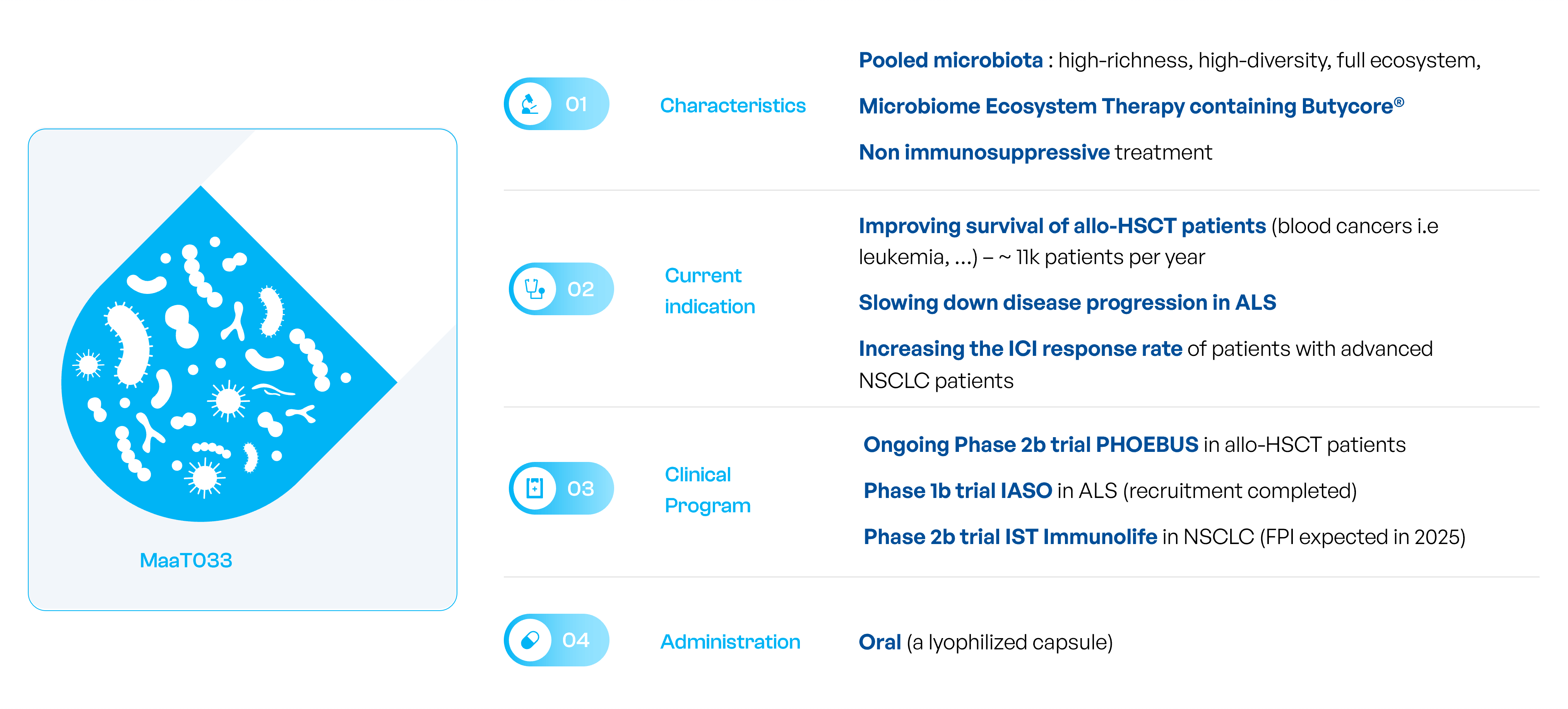
MaaT033, the oral ecosystem microbiome capsule for adjunctive therapy
The unmet need
Allogeneic Hematopoietic Stem Cell Transplantation (allo-HCT) is a potentially life-saving procedure for people suffering from blood cancers such as acute myeloid leukemia.
Although potentially life-saving, allo-HCT can be associated with severe complications, such as severe infections, graft-vs-host-disease, neutropenia, contributing to a mortality rate of 34% after 1 year6 in patients over 50 years of age receiving allo-HCT.
20,000
MaaT033 to improve outcomes in patients receiving allo-HCT
Antibiotic and chemotherapy-derived loss of gut diversity is predictive of mortality in allo-HCT patients, as well as graft-vs-host-disease occurrence and infection.2 3 4 7
MaaT033 is a an oral, donor-derived, standardized, high-richness, high-diversity microbiome ecosystem therapies, for ambulatory use, containing ButycoreTM , a group of bacterial species known to produce anti-inflammatory short-chain-fatty acid. MaaT033 has received Orphan Drug Designation from the EMA.
- Restoration of the full richness and diversity of a healthy microbiome and production of immune-regulating short-chain-fatty-acids known to upregulate Treg cells activity, in order to restore immune homeostasis,
- Restoration of the gut barrier, to prevent infections, especially those related to multi-drug-resistant bacteria (MDRB).
MaaT033 status
In June 2022, MaaT Pharma reported positive results for MaaT033 in Phase 1b clinical trial for patients with acute myeloid leukemia including a good microbiome engraftment and engraftment persistence, together with a satisfactory safety profile for its second drug-candidate.
Additional information:
- Clinical trial: NCT04150393
- Press release announcing Phase 1b CIMON clinical results
- Access the poster presented during the 64th edition of the American Society of Hematology
Ongoing Phase 2b trial
In November 2023, MaaT Pharma announced that the first patient has been treated as part of its Phase 2b trial, called PHOEBUS, investigating the efficacy of MaaT033 in improving overall survival (OS) at 12 months for patients with blood cancer receiving allogenic Hematopoietic Stem Cell Transplantation. The trial is an international, multi-center, randomized, double-blind, placebo-control study (NCT05762211), which will be conducted in up to 56 clinical investigation sites and is expected to enroll 387 patients. It is to date the largest randomized controlled trial assessing a microbiome therapy in oncology.
MaaT033 to restore gut microbiome richness and diversity for patients with ALS
The unmet need
Recent studies have has started to shed light on the role of the gut microbiome, linking abnormalities to diseases such as Amyotrophic lateral sclerosis (ALS[8]). The link between gut microbiota and ALS first emerged from preclinical evidence and then from clinical observations indicating a disease-modifying role for the gut microbiome.
To date, there is no effective treatment for ALS, a disease that leads to death within on average 3-5 years after diagnosis[9]. Since its inception, MaaT Pharma has been committed to restoring a microbiome symbiosis in life-threatening diseases with high unmet clinical needs. Growing evidence suggests that ALS patients show increased inflammation in the gut and changes in the composition of gut microbes, with low levels of beneficial bacteria.
60,000
MaaT033 to slow down disease progression
MaaT Pharma has decided to extend its scientific research to the management of ALS, which could pave the way for the treatment of several neurodegenerative diseases. To do so, the Company is leveraging the strong safety profile of its native MET products (MaaT033/MaaT013), inherent product characteristics of promoting immune modulation/anti-inflammatory properties and acting as a homeostasis hub.

- Up to 15 patients in a pilot, open-label, Phase 1b study in France
- Key study endpoints: assess safety and tolerability of MaaT033 and gut microbiota composition evolution

This pipeline expansion to a new indication demonstrates the strong potential of MaaT033 to be used in acute or in chronic conditions as a standalone, adjunctive therapy.
ClinicalTrials.gov Identifier: NCT05889572
Sources
1 Castilla-Llorente. C, et al. Prognostic factors and outcomes of severe gastrointestinal GVHD after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2014 49(7):966-71. doi:10.1038/bmt.2014.69
2 Peled JU, et al. Role of the intestinal mucosa in acute gastrointestinal GVHD. Blood. 2016 128(20):2395-2402. doi: 10.1182/blood-2016-06-716738.
Staffas A, et al. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood. 2017 129(8):927-933. doi: 10.1182/blood-2016-09-691394. Erratum in: Blood. 2017 Apr 13;129(15):2204
Mathewson ND, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol. 2016 505-513. doi: 10.1038/ni.3400. Epub 2016 Mar 21. Erratum in: Nat Immunol. 2016 Sep 20;17 (10 ):1235.
3 Taur Y, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014 124(7):1174-82. doi:10.1182/blood-2014-02-554725.
Jenq RR, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012 903-11. doi: 10.1084/jem.20112408.
4 Jenq RR, et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2015 1373-83. doi:10.1016/j.bbmt.2015.04.016.
Peled JU, et al. Intestinal Microbiota and Relapse After Hematopoietic-Cell Transplantation. J Clin Oncol. 2017 1650-1659. doi:10.1200/JCO.2016.70.3348
5 Global Data GVHD Epidemiology Report, Jan 2020.
6 EBMT data 2021
7 Malard, F., Vekhoff, A., Lapusan, S. et al. Gut microbiota diversity after autologous fecal microbiota transfer in acute myeloid leukemia patients. Nat Commun 12, 3084 (2021). https://doi.org/10.1038/s41467-021-23376-6
[8] Rowin et al., 2017; Nicholson et al, 2021; Blacher et al, 2019, Mazzini et al, 2020
[9] https://tousensellescontrelasla.fr/la-sla-cest-quoi/
MaaT03X to boost cancer therapy
The unmet need
Immune checkpoint inhibitors (ICI) have proved a formidable therapeutic option for the treatment of solid tumors. They have notably improved survival in more than 15 oncology indications to date. However, not all patients respond to ICI. In some indications, it has been suggested that the gut microbiome plays a role in the response to ICI. Recent research has suggested that a patient’s gut microbiome diversity and richness, as well as its composition, impacts response rate to ICI and overall survival rate in multiple indications (melanoma, non-small cell lung cancer, renal cell carcinoma…).
80%
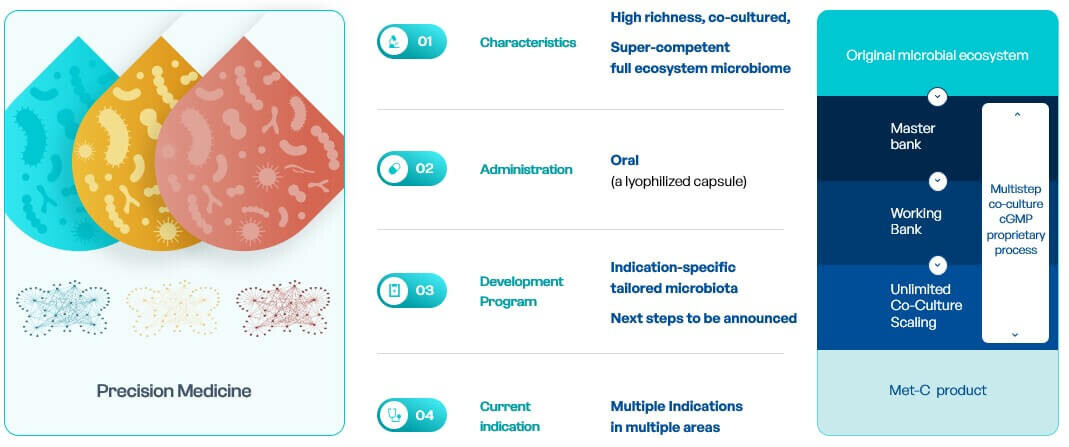
MaaT034 to optimize immune checkpoint inhibitor treatment
MaaT034, the first-in-class co-cultured product, is aiming at optimizing intestinal microbiome functions prior to and during immunotherapy in major oncology indications, and become a (neo)-adjuvant therapy across ICI therapies, leading to better survival in cancer patients. The objective of MaaT Pharma is to develop MaaT034 as tumor-agnostic adjunctive therapies to ICI (i.e. administration before and in combination with ICI whatever the type of cancer) leading to improved clinical outcomes and lower incidence of complications than using ICI alone.
To achieve this, MaaT Pharma will capitalize on MET-N products: an Investigator-Sponsored Trial (IST) is already ongoing in solid tumors with the PICASSO trial (metastatic melanoma, with MaaT013) to evaluate the safety of MET-N products in combination with ICI and collect preliminary efficacy data assessing the impact of MET products on the immune system and anti-tumoral responses.
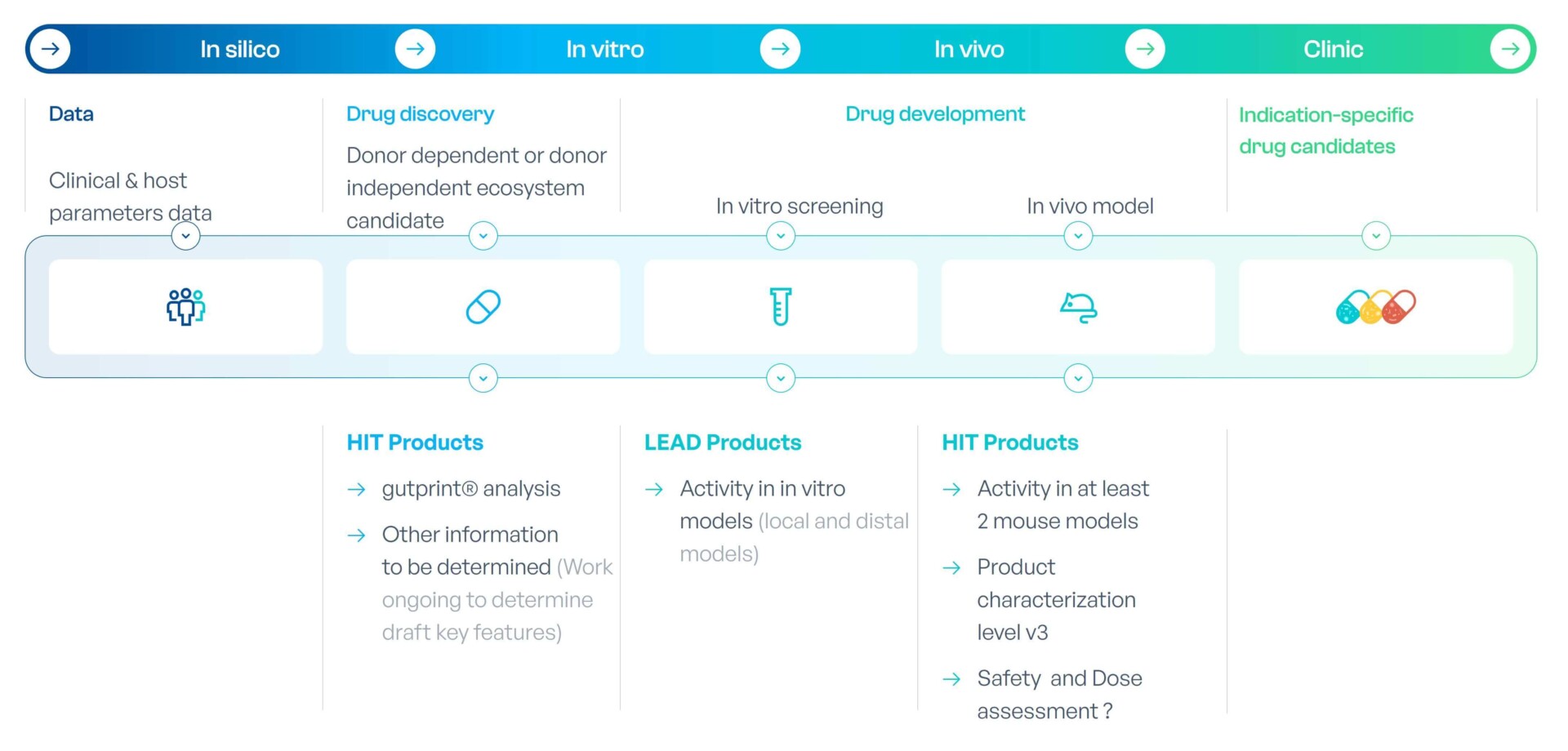
MaaT03X, a Tailor-made AI-driven Super-Competent Synthetic Microbiota for specific indications
MaaT03X are indication-specific tailored super-competent co-cultured microbiome ecosystem. Through the collection of data from patients, healthy donors, literature, and data during the development of MaaT034 (fecal and biological samples), MaaT Pharma will feed the gutPrint AI platform allowing to create the next-generation microbiome therapies.
Scientific publications
Article & review
- Biennier S. et al (2024). Narrative Review: Advancing Dysbiosis Treatment in Onco-Hematology with Microbiome-Based Therapeutic Approach. Microorganisms
- Laperrousaz B. et al (2024). Safety comparison of single-donor and pooled fecal microbiota transfer product preparation in ulcerative colitis: systematic review and meta-analysis. BMC Gastroenterology
- Reygner J. et al (2024). Reduction of product composition variability using pooled microbiome ecosystem therapy and consequence in two infectious murine models. Applied and Environmental Microbiology- ASM Journals
- Malard F. et al. (2023).Pooled allogeneic faecal microbiota MaaT013 for steroid-resistant gastrointestinal acute graft-versus-host disease: a single-arm, multicentre phase 2 trial. eClinicalMedicine
- Malard F. et al. (2021). Restoration of gut microbiota diversity with autologous fecal microbiota transfer in acute myeloid leukemia patients. Nature communications.
- Malard F. et al. (2018). High gastrointestinal microbial diversity and clinical outcome in graft-versus-host disease patients. Bone Marrow Transplantation.
Posters
- Malard F. et al (2024). MaaT033 to ensure optimal intestinal microbiota to improve survival of patients receiving allo-HSCT: PHOEBUS trial. SFGM-TC Congress (French only)
- Malard F. et al (2024). PHOEBUS Trial: an international, randomized, double-blind, multicenter phase IIb study evaluating MaaT033, oral allogeneic fecal microbiotherapy, in patients undergoing allo-HSCT to improve overall survival. SEHH Congress (Spanish only)
- Malard F. et al. (2022). Pooled allogenic fecal microbiotherapy MaaT013 for the treatment of steroid-refractory gastrointestinal acute graftversus-host disease: results from the phase IIa HERACLES study and expanded access program. IHMC congress
- Gasc C. et al. (2022). Pooling of faecal material results in standardized and high-richness microbiotherapy products MaaT013 and MaaT033. IHMC congress
- Duquenoy A.et al. (2022).Development of a culture manufacturing process preserving the profile of gut microbiota for therapeutic purposes. IHMC congress
- Malard F. et al. (2019). Successful and safe treatment of intestinal Graft-versus-Host Disease (GvHD) with pooled-donor full ecosystem microbiota biotherapeutics. Blood. ASH congress
- Malard F. et al. (2019). Heracles: a phase II single-arm prospective study to assess the efficacy of fecal microbiota transfer (FMT) in the treatment of steroid-refractory gastro-intestinal predominant aGVHD post allo-HSCT. EBMT congress
- Mohty M. et al. (2018). The Odyssee Study: Prevention of Dysbiosis Complications with Autologous Fecal Microbiota Transfer (FMT) in Acute Myeloid Leukemia (AML) Patients Undergoing Intensive Treatment: Results of a Prospective Multicenter Trial. Blood. ASH congress
- Mohty M. et al. (2017). Prevention of dysbiosis complications with autologous fecal microbiota transplantation (auto-FMT) in acute myeloid leukemia (AML) patients undergoing intensive treatment (ODYSSEE study): first results of a prospective multicenter trial. Blood. ASH congress
Oral communications
- Malard F. (2024) Pooled fecal allogenic microbiotherapy for refractory gastrointestinal acute graft-versus-host disease: results from the early access program in Europe. SFGM-TC Congress
- Sanz J. (2024) Allogeneic fecal microbiotherapy as a treatment for refractory Graft-versus-Hostacute gastrointestinal disease: results of the EAP in Europe. SEHH Congress
- Malard F. et al. (2021). 262 Pooled Allogenic Fecal Microbiotherapy MaaT013 for the Treatment of Steroid-Refractory Gastrointestinal Acute Graft-Versus-Host Disease: Results from the Phase IIa Heracles Study and Expanded Access Program. ASH congress
- Malard F. et al. (2021). Successful and Safe Treatment of Intestinal Graft-Versus-Host Disease (GvHD) with Pooled-Donor Full Ecosystem Microbiota Biotherapeutic: Results from a 29 Patient-Cohort of a Compassionate Use/Expanded Access Treatment Program. EBMT congress
- Malard F. et al. (2020). Successful and Safe Treatment of Intestinal Graft-Versus-Host Disease (GvHD) with Pooled-Donor Full Ecosystem Microbiota Biotherapeutic: Results from a 29 Patient-Cohort of a Compassionate Use/Expanded Access Treatment Program. Blood. ASH congress
- Malard F. et al. (2020). Successful and safe treatment of intestinal graft-versus-host disease (GvHD) with pooled-donor full ecosystem microbiota biotherapeutics. EBMT congress
- Malard F. et al. (2019). The odyssee study: prevention of dysbiosis complications with autologous fecal microbiota transfer in acute myeloid leukemia patients undergoing intensive-treatment: results of a prospective multicenter trial. EBMT congress