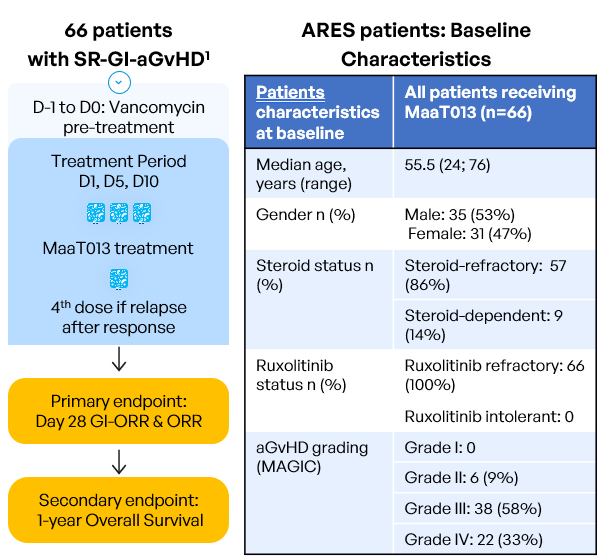
Inclusion criteria:
- Age ≥ 18 years old
- Allo-HSCT with any type of donor, stem cell source, GvHD prophylaxis or conditioning regimen
- Patients who develop aGvHD episode with GI involvement per MAGIC guidelines (=grades II to IV), with or without involvement of other organs (Harris et al. 2016)
- Patients resistant to steroids AND either resistant to OR with intolerance to ruxolitinib (intolerant patients: who had grade 3 or higher treatment-emergent and ruxolitinib-attributed adverse event that did not resolve within 7 days of discontinuing ruxolitinib). The diagnosis must be confirmed within 48h prior to study pre-treatment start.